Actinium-225 (Ac-225), 136Ac89, is an important radioisotope in nuclear medicine with applications in the targeted alpha therapy (TAT) in oncology. Ac-225 contains 89 protons and 136 Neutrons, corresponding to atomic mass of 225 amu (1 amu ~= 1.66 x 10⁻²⁷ kg). It is the first element in the Actinide Series of the periodic table, a group of fifteen rare earth metal elements, that also include Uranium and Plutonium. The decay chain of Ac-225 is represented in Figure 1.
Figure 1: Ac-225 decay chain
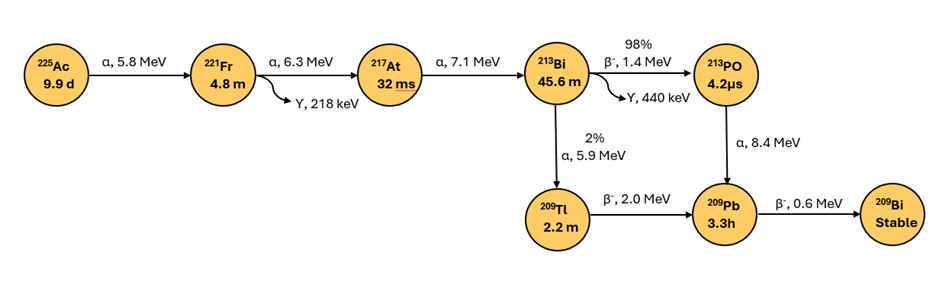
Ac-225 decays through a series of six short-lived radioactive daughter isotopes to stable Bismuth-209 (Figure 1) via two pathways with major decay proceeding via Po-213 . Ac-225, the parent radioisotope, is the radioisotope with the longest half-life in the decay chain at 9.9 days. Longer half-life of Ac-225 allows for longer therapeutic effect compared to short lived radioisotopes while also requiring longer storage time for radioactive manufacturing waste streams prior to disposal. Ac-225 therapy typically presents low external radiation concerns from patients due to short penetration range of α particles, and safety of patients, caregivers and family is handled per protocols provided by healthcare providers.
Either decay pathway releases four α particles (energy range 5.8-8.4 MeV) and two β particles (energy range 0.6-2 meV) per decay event, highlighting α emission as the major decay mode. In addition, two γ emissions, 218- 440 keV, occur per decay event as shown by the wavy arrows in Figure 1. Comparatively, Tc99m based SPECT imaging agents emit γ rays of 140 keV energy. As Ac-225 emits γ rays, patients could be imaged during the Ac-225 treatment to monitor the progress of the treatment, with safety precautions appropriated for higher energy radiation.
Characteristics of α particles and clinical implications
α and β– radiations (e.g. from Lu-177) are two types of ionizing radiations that play a significant role in the targeted radioligand therapy (TRLT) in oncology. While both have the ability to damage cancer cells, their characteristics lead to differences in the mechanism of action and therapeutic applications as described below.
α particles are extremely heavy (atomic mass 4 amu, 1 amu = ~ 1.66 x 10⁻²⁷ kg) relative to β– particles (~1/2000 amu). α particles are larger in size (diameter ~ 3.6 x 10⁻¹⁵ meters) whereas β– (hereon referred to β) particles are essentially considered point particles with no measurable diameter.
Regarding travel distance of α particles in cellular environment, understanding of average sizes of human cells is necessary. Human cell vary considerably in size depending on the type and function. Most human cells are generally 10-100 µm in length and average cells are typically 10-30 µm in diameter. Diameters of some cells, for example, are: red blood cells ~ 8-9 µm, skin cells ~ 10-30 µm, pancreatic beta cells ~ 10-15 um, prostate luminal cells ~ 10-15 µm.
Because of higher mass and large size, α particles travel typically 50-100 µm in tissues, corresponding to 3-10 cell lengths based on the average cell diameter. Comparatively, travel range of beta particles is considerably longer: a 0.8 MeV beta particle can travel up to 1-2 mm or 1000-2000 µm in cellular environment, or 10-20 times the range of alpha particles.
Alpha particles are higher energy, typically in the range of 5-9 MeV. Due to high energy and a short travel range, α particles induce high localized damage to cancer cells while minimizing harm to surrounding healthy tissues (lesser “bystander” effect). Due to their high “momentum” energy, α radiation causes double-strand DNA breaks, making it highly cytotoxic. Comparatively, Beta radiation causes single-strand DNA breaks.
The limited penetration range coupled with high energy makes α emitters suitable for treating localized cancers, for post-surgery treatment, and micro-metastases, small clusters of cancer cells that have spread from a primary tumor to other parts of the body.
Ac-225 related clinical trials
As of June 2025, there are no FDA or EMA approved Ac-225 radiotherapies, however, few targeted radiopharmaceuticals involving Ac-225 are currently in clinical trials. The number of such clinical studies, listed in the table below, are under 10, a rather limited number. Majority of the studies are for the treatment of prostate cancer.
Table 1: Clinical Trials involving Ac-225
| Clinical trial reference | Indication | Clinical phase | Biomarker | Organization |
| 225Ac-PSMA-617 (NCT04597411) | Prostate cancer | Phase 1, recruiting patients | PSMA | Novartis |
| AAA802 (225Ac-PSMA-R2) (NCT05983198) | Prostate cancer | Phase 1, recruiting patients | PSMA | Novartis |
| AAA817 (225Ac-PSMA-617) (NCT06780670) | Metastatic castration-resistant prostate cancer (mCRPC) | Phase 2/3, Recruiting patients | PSMA | Novartis |
| Actimab-A with CLAG-M chemotherapy regimen | Relapsed/Refractory Acute Myeloid Leukemia (AML) | Phase 2/3 planned | CD33 | Actinium Pharmaceuticals |
| Actimab-A with Hypo Methylating Agents Venetoclax and ASTX-727 (NCT06802523) | Newly Diagnosed AML | Phase 1 planned | CD33 | Actinium Pharmaceuticals |
| TLX592 (225Ac-RADmAb®) (CUPID study) | Prostate cancer | Phase 0/I | PSMA | Telix Pharmaceuticals |
| AZD 2284 (D7580C00001) | Prostate cancer | Phase 1 in progress | STEAP2 (six-transmembrane epithelial antigen of prostate-2) | Astra Zeneca |
| FPI-2265, TATCIST trial (NCT05219500) | Prostate cancer | Phase 2 in progress | PSMA | Astra Zeneca |
The low number of Ac-225 clinical trials reflect, in part, challenges in the Ac-225 isotope supply, as development of manufacturing processes for producing clinical and commercial quantities of Ac-225 are still in progress. However Ac-225-PSMA-617 has been used under Compassionate use category, also called expanded access, outside of clinical trials, typically for patients with no other treatment options. Early compassionate use cases of Ac-225-PSMA-617 have shown high PSMA declines, ≥ 90% in some patients, and tumor responses in patients resistant to Lu-177 therapy.
