Prostate cancer is one of the most prevalent cancers among men worldwide. Targeted PET imaging in prostate cancer plays a vital role in detecting metastasis to guide treatment decisions.
In the last few years, three radiotracers for prostate cancer, Pylarify (Piflufolastat F-18), Illuccix (Ga-68 PSMA-11) and Posluma (Flotufolastat F-18), have been approved by FDA. These tracers target PSA (prostate-specific antigen) biomarker, a protein overexpressed in prostate cancer cells. These tracers are indicated for prostate cancer patients with suspected metastasis and biochemical recurrence (BCR) based on elevated serum PSA levels.
PSA is overexpressed in ~ 90% of prostate cancers, with minimal presence in benign cells. PSA targeted tracers have revolutionized prostate cancer imaging by enabling better accuracy in the detection of cancer at low PSA levels compared to conventional imaging.
Clinical trials of radiotracers
Clinical trial references for the three radiotracers, where the data for the calculation of imaging performance parameters was generated, are provided below:
. Illuccix (Ga-68 PSMA-11): FDA approved Illuccix based on the VISION Phase III study (NCT03511664) for the detection of prostate cancer and to identify patients for the PSMA-based radioligand therapy.
. Pylarify (Piflufolastat F-18): The safety and efficacy of Pylarify were evaluated in two clinical trials with a total of 593 men with prostate cancer. In the first trial (NCT02981368), 268 patients with biopsy-proven prostate cancer, considered at higher risk of metastasis, underwent Pylarify PET/CT scans. The second trial (NCT03739684) enrolled 208 patients with biochemical evidence of recurrent prostate cancer.
. Posluma (Flotufolastat F-18): The FDA approved Posluma based on the two clinical trials, NCT04186819 and NCT04186845, where a total of 747 patients with prostate cancer participated. First clinical trial included 356 patients with newly diagnosed prostate cancer and second clinical trial included 391 patients with the suspicion of recurrent cancer indicated by rising PSA.
Prior PET tracers
Prior to PSMA-targeted tracers, PET tracers in prostate cancer used nonspecific tracers F-18 fluciclovine (Axumin) and C-11 Choline. F-18 fluciclovine is a synthetic amino acid that is taken up in prostate cancer cells to a higher extent compared with surrounding normal tissues. The uptake mechanism of 11C-choline tracer in cells is based on its role as a precursor for the synthesis of phospholipids, a crucial component of cell membranes.
Comparing diagnostic performance of Imaging Agents
There are several ways to compare diagnostic radiotracers. Sensitivity and specificity are appropriate for comparing diagnostic performance as they directly reflect how well a tracer can detect cancer (sensitivity) and how well it rules out the cancer when absent (specificity).
About F-18 and Ga-68 tracers
PET tracers with different radioisotopes have different energies associated with associated positrons: F-18 emits positrons of about 0.6 MeV and 0.25 MeV maximum and average energies respectively, whereas Ga-68 emits positrons of about 1.9 MeV and 0.8 MeV maximum and average energies respectively. As positron energy influences image resolution, theoretically there is a difference in image resolution between F-18 Vs. Ga-68 tracers, with higher resolution favored for F-18 tracers due to its lower energy positrons: F-18 positrons travel lesser distance in tissues before annihilation relative to Ga-68 positrons, theoretically providing higher resolution images.
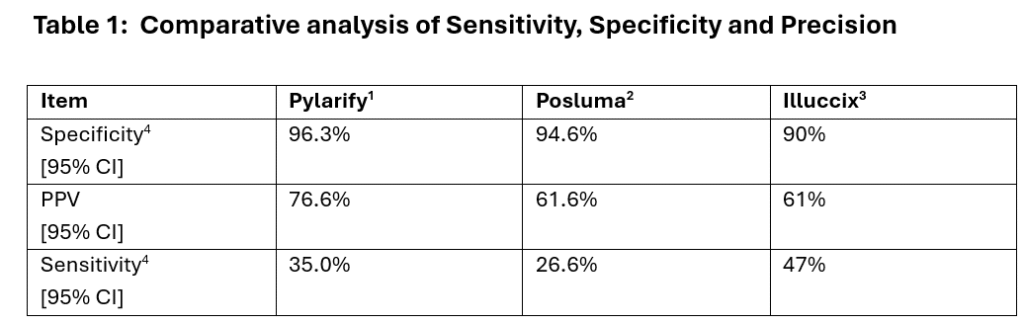
Table below provides sensitivity, specificity as well as PPVs (Positive Predictive Values), or precision where PPV is defined as
True Positives / (True Positives + False Positives)
1: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214793s000lbl.pdf
2: https://www.posluma.com/prescrirbing-information.pdf
3: https://illuccixhcp.com/wp-content/uploads/illuccix-prescribing-information.pdf
4. Values calculated are from references 1-3 and are averages of three panelists that reviewed images.
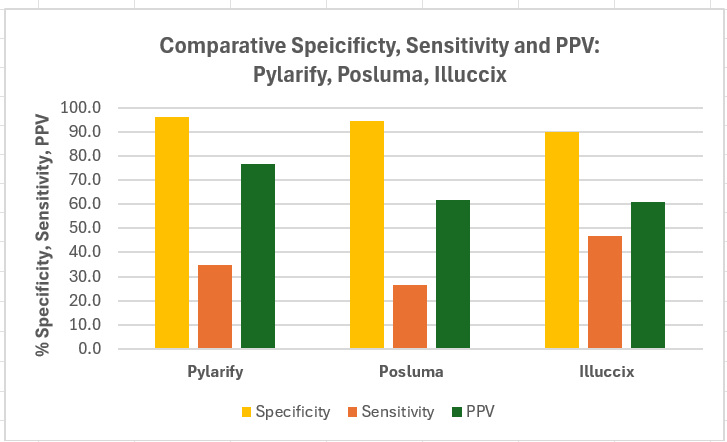
Chart below provides a visual comparative representation of above parameters for the three tracers.
Analysis of Sensitivity & Specificity
Sensitivity: All three tracers showed lower sensitivity (35%–47%). The relatively low sensitivity may be partly due to: (a) a small percentage of prostate cancer cells may not express PSMA; (b) PSMA PET scans have limitations in detecting lesions below certain size, therefore some micrometastatic lesions may go undetected; (c) high uptake of the tracer in certain normal tissues like kidneys or liver could obscure lesions in these areas due to high background noise level; (d) tracer uptake may not be specific to PSA expressing cells.
There’s typically an inverse relationship between sensitivity and specificity many diagnostic tests, including in the present case, and increased specificity comes at the expense of lower sensitivity. In clinical setting, high sensitivity is desired in screening tests to minimize false negatives and avoid missing potential cases of a disease. As the three tracers we are discussing are approved for the detection of metastatic disease, and not used for screening for prostate cancer, low sensitivity of these tracers can be worked with.
Specificity: The three tracers exhibited high specificity (90-96%) and demonstrate confidence in lower false negatives. Pylarify, Illuccix, and Posluma show smaller differences in the specificities with Pylarify demonstrating higher specificity.
Radiotracer Market
The global radiopharmaceutical market is rapidly growing, driven by advances in radiodiagnostics, radiotherapeutics as well as aging populations. An eReport on the radiodignostics market including prostate cancer radiotracers for 2024 and future years is available (https://pharmafronts.com/product/global-theranostic-radiopharmaceuticals-market-2024/).
