This blog discusses radiohybrids, theranostics and theranostic pairs. Radiohybrids is a modality in nuclear medicine that allows diagnostic imaging or therapeutic treatment with the same molecular entity depending on the radioelement in the molecule.
POSLUMA (flotufolastat F 18) injection (Bracco, Blue Earth Diagnostics), approved as a prostate cancer specific PET imaging agent by FDA in May 2023, is an example of a radiohybrid.
The active radioelement in POSLUMA is F-18; it also contains chelator bound nonradioactive Ga3+: F-18 is covalently bound and Ga3+ is bound via a DOTA chelator.
A schematic of a radiohybrid is shown in Figure 1 below.
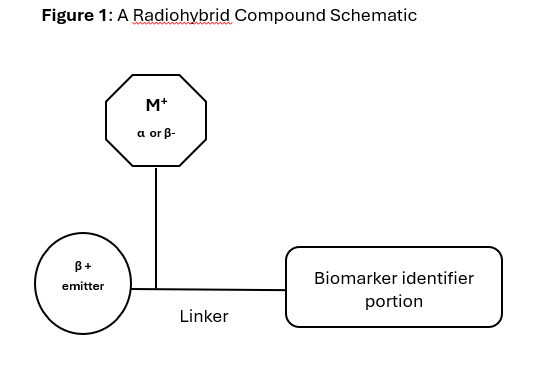
A radiohybrid, as exemplified by Posluma (flotufolastat F 18), represents a cutting-edge class of radiopharmaceutical, specifically designed for both diagnostic imaging and potential therapeutic applications in the context of theranostics.
It’s a specially engineered molecule with two designated binding sites for different radioisotopes: one for an imaging isotope (like fluorine-18, or F-18) and another for a therapeutic isotope (like lutetium-177, or Lu-177). Key aspects of radiohybrid technology are:
- Dual labeling capability: Radiohybrids can be labeled with either an imaging radionuclide e.g. F-18, Cu-64 (for PET scans) or a therapeutic radionuclide e.g. Lu-177, Ac-225 for targeted radiation therapy.
- Identical chemical structure: Regardless of whether radiohybrid is used for imaging or therapy, the chemical structure retains the same. This means the compound’s pharmacokinetic and pharmacodynamic behavior remains same (e.g., binding to target biomarker, distribution and elimination in the body).
- Theranostic ability: Dual purpose modality: Imaging version of the radiohybrid may be used to first visualize the target (e.g., prostate cancer cells), then therapeutic version can be administered ensuring that radiation will be delivered directly to the same lesions visible on the PET scan.
Theranostics, also called theragnostics is a term used with radiopharmaceuticals. The word comes from the combination of diagnostics and therapeutics. It essentially is used to describe both radiodignostic and radiotherapeutic products.
Theranostic pairs are sets diagnostic imaging product and a radiotherapeutic product. The molecular structure of this pair is slightly different as shown in Figure 2 below.
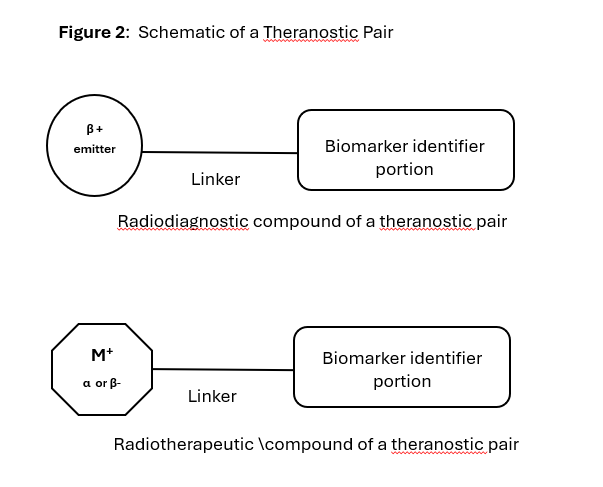
FDA has approved theranostic pairs for some therapies. E.g. Lutathera (lutetium Lu 177 dotatate), used for treating somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs) tumors had “Netspot” approved as an imaging agent prior to therapy. It is a PET diagnostic agent based on Ga-68 that images GEP-NET tumors expressing somatostatin receptors.
In summary, this blog discussed radiohybrids, theranostics and theranostic pairs illustrative examples.
