Introduction
Mayo Clinic dosed the first patient last week with Ac-225-DOTATATE radiotherapeutic (RYZ101), an alpha-emitter, by RayzeBio (a BMS company), for metastatic, estrogen receptor–positive (ER⁺), human epidermal growth factor receptor negative (HER2-) breast cancer. This is an open-label, multicenter Phase 1b/2 trial (TRACY-1).
177Lu-DOTATATE, lutetium -177 oxodotreotide (Lutathera), is a β– particle emitting radiotherapy approved by FDA in 2018 for patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs) that are SSTR+, and has been proved effective based on its commercial success-in 2024, it generated approximately USD 724 million in sales.
Clinical investigations for somatostatin based SSTR2 biomarker is accelerating, and this article looks into structural analysis of synthetic analogs of natural somatostatins that play a crucial role in these therapies.
Natural somatostatin and somatostatin receptors
Somatostatins are natural hormones produced mainly by pancreatic cells, neurons in hypothalamus and gastrointestinal neuroendocrine cells. They bind to somatostatin receptors expressed on various cells to regulate critical processes including cell growth regulation.
Somatostatin receptors have five subtypes (SSTR1–5), of which SSTR2 is most frequently overexpressed in certain cancers. E.g., GEP-NETs express SSTR2 in 80–90% of cases which led to the development of SSTR2 based radiotherapy, illustrated by Lutathera.
Binding of somatostatin analogs to SSTR2 permits both targeted imaging and targeted therapy. E.g., Ga-68 DOTATATE has been approved as a diagnostic aid for Lutathera therapy, a “theranostic pair”.
Radiolabeled somatostatin analogs bound to SSTR2 are internalized via endocytosis and deliver radiation close to tumor cell nucleus to induce single or double-strand DNA breaks, while minimizing damage to healthy tissue.
Synthetic Somatostatin Analog: DOTA-TATE
Lutathera and RYZ101 both use DOTA-TATE, an eight-amino-acid somatostatin analog optimized for SSTR2 binding and increased stability in plasma (half-life of several hours); it contains DOTA chelator for radiometal binding and a cyclic peptide for SSTR2 binding. These characteristics enable effective imaging and therapy.
Structure of somatostatin analogs
Lutathera is a synthetic somatostatin, with structure shown in Figure 1 (Ref.).
Figure 1: Chemical structure of Lutathera
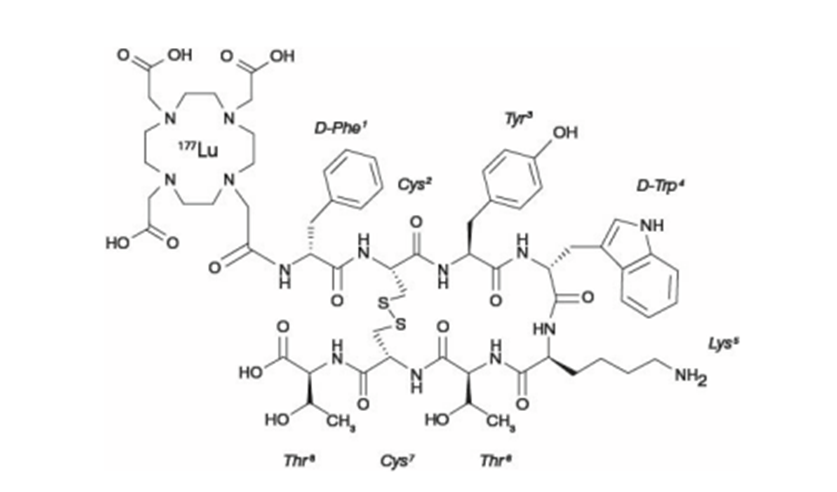
The structure contains DOTA chelator (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid ) that binds the radiometal, a linker, and the cyclic octapeptide containing a disulfide bridge that binds to SSTR2. The peptide component sequence is as follows:
D-Phe–Cys–Tyr–D-Trp–Lys–Thr–Cys–Thr
The cyclopeptide has two unnatural amino acids, D-phenylalanine and D-tryptophan.
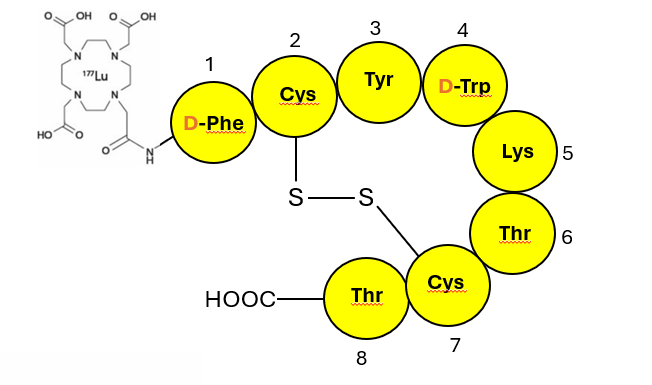
The schematic of Lutathera is shown in Figure 2, a simplified visual for clarity. The DOTA chelator is at the N terminal of the peptide backbone attached via a short linker; the unnatural D-amino acids in the peptide portion have been identified.
Figure 2: Schematic of Lutathera, a cyclo octa-peptidic synthetic somatostatin
For comparison purpose, a schematic of 14-amino acid natural somatostatin is shown in Figure 3 (Ref.). (one of the two natural forms shown; 28-amino acid cyclic peptide form not shown)
Figure 3: Schematic of natural somatostatin, a cyclo tetra-deca peptide
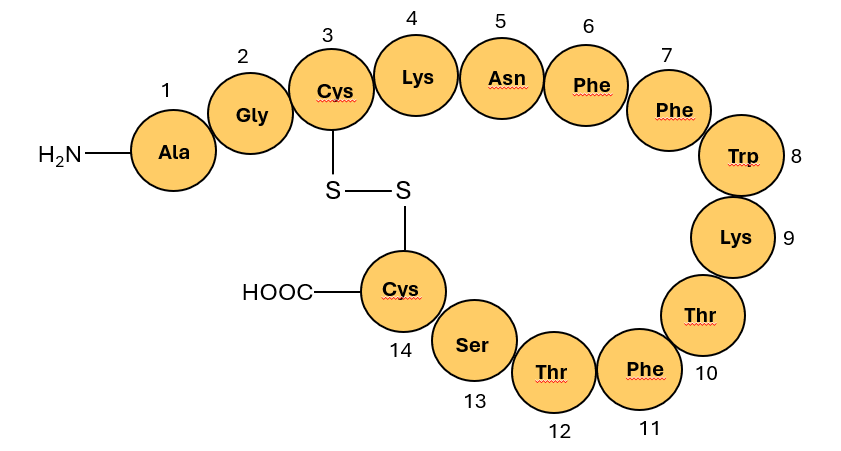
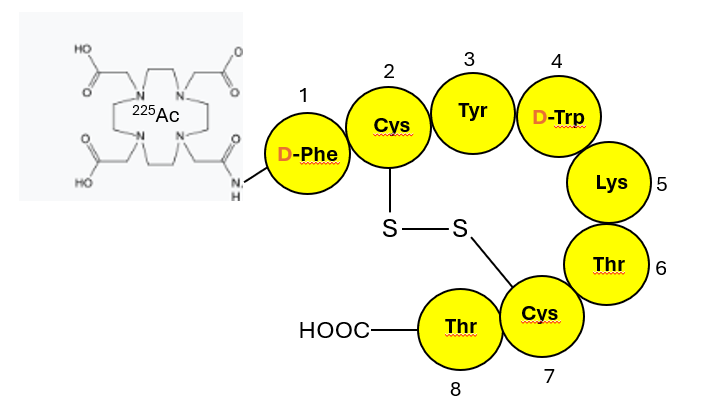
Figure 4 shows the chemical structure of RYZ 101, the breast cancer investigational candidate mentioned previously, which is identical to Lutathera except that radiometal Ac-225 has been substituted for Lu-177.
Figure 4: Schematic of RYZ 101, a cyclic octa-peptidic synthetic somatostatin
BMS is also currently enrolling patients for a Phase 3 global randomized clinical trial for GEPNETs, investigating RYZ101 against patients developing resistance to Lutathera therapy (“ACTION-1”, NCT05477576) (Ref.)
Additional Clinical Indications
Somatostatin receptor–targeted therapies are being investigated for several other indications e.g., extensive-stage small cell lung cancer, pancreatic NETs, bronchial NETs, lung NETs, illustrating wider scope of this treatment modality.
Conclusion
Somatostatin biomarker–based radiotherapies exemplify precision oncology’s power to exploit tumor-specific receptors. Lutathera’s success in GEP-NETs established the clinical value of SSTR2 targeting, while RYZ101’s first patient dosing in breast cancer heralds an alpha-emitter modality for a new, widely applicable indication. As additional investigations expand into lung and other SSTR2⁺ cancers, radiotheranostics provide potential for improved disease treatments in the near future.
